Peer Reviewed Article
Abstract
Over the past several years, a new corrosion control technology has been developed for protecting damaged, painted galvanized and galvannealed surfaces in contact with ambient aqueous environments. This technology, which we call Electromagnetically Induced Corrosion Control Technology (EICCT), is an electronic technology that is based upon coupling surface currents into the metal structure to be protected. Electromagnetic induction experiments have demonstrated that the induced current is spread across the surfaces of complex shapes, such as an automobile body, as required by Maxwells equations, so that induction at a single point is effective in protecting a whole, complex-shaped surface, that the power consumption is very low, and possibly that the induced signal may be tailored to optimize the efficacy. The observed efficacy is attributed to inhibition of zinc passivation as directly indicated by the coupling current. Efficacy is also indicated by inhibition of rusting at scribes in painted panels. It is important to emphasize that the technique is not a classical, impressed current cathodic protection (ICCP) system.
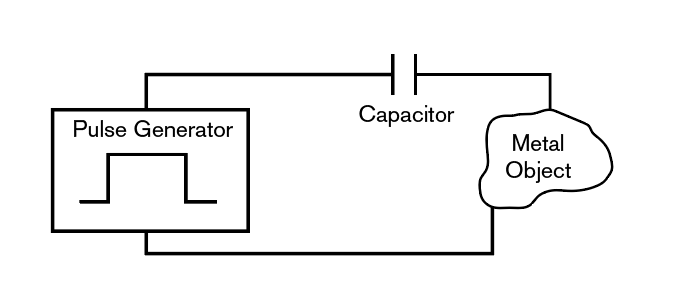
The Module Output
The Module consists of a pulse voltage source capacitively coupled to the metallic object to be protected. The pulse voltage source generates a voltage pulse with:
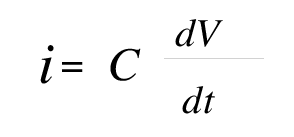
Voltage Pulse
The pulsed voltage source is coupled to the body to be protected through a capacitor as shown. The capacitor essentially differentiates the voltage pulse and a pulsed current flows through the circuit, which includes the body to be protected. The relevant equation is
where i is the current, V is the applied voltage and t is time. This current pulse is very fast and flows primarily in the surface of the metal. The current is constrained to the surface of the metal as a consequence of the skin effect, the skin depth in zinc is 100 µm. and within steel it is 16µm. Clearly, then most of the current flows in the surface. Corrosion being a surface phenomenon, these currents are present at the right place to interfere with the corrosion process.
Spray Experiments
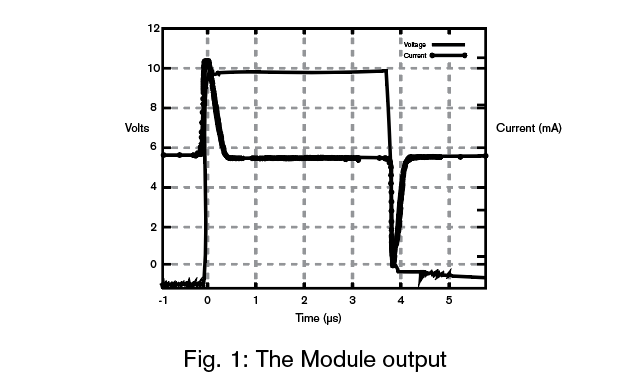
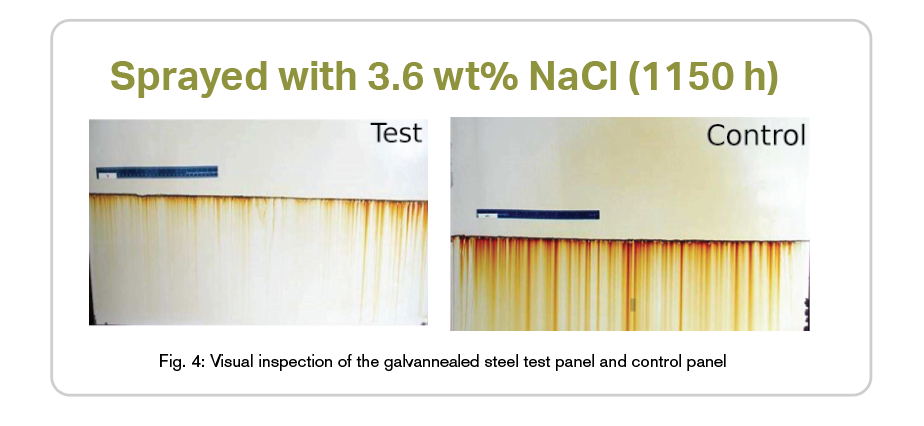
In this set of experiments, three of the five experiments used two automobile stock galvannealed steel panels per experiment, each measuring 1.22 m. by 0.914 m.,which were coated on both sides with DUPONT 72400 by Northwest Campus Auto Body located in Columbus, Ohio. This is a standard coating system for exterior automobile bodies and the test efficacy was indicated by the ability of the Module to displace the corrosion potential at the scribe. The panels were scribed to cut through the clear coat, paint, and galvannealed layer, thereby exposing the bare steel. A reference electrode was placed at the location of the scribe on each panel to monitor the corrosion potential of each panel. Both panels were inclined at 25 degrees to the vertical with the scribed surface facing outward and with the scribe being located approximately 3 feet down from the top edge.
Configuration of the Test Panel
The scribed surfaces of the panels were continuously sprayed with a 3.6 wt.% NaCl (salt) solution to simulate road salt exposure. Only the surface of the panel in the vicinity of the scribe, and the surface below, was inundated with the electrolyte; the remainder of the panel surface remained dry. In fact, the distance between the top of the spray zone and the location where the Module was connected to the Test Panel was approximately 2 feet showing that any possibility of a return electrolyte path can be discounted.
Summary
The work described in this paper was designed to evaluate the efficacy of electromagnetically-induced surface currents in reducing the development of corrosion damage on galvanized and galvannealed steel panels and to indicate, to the extent possible, the mechanism by which protection is accomplished.